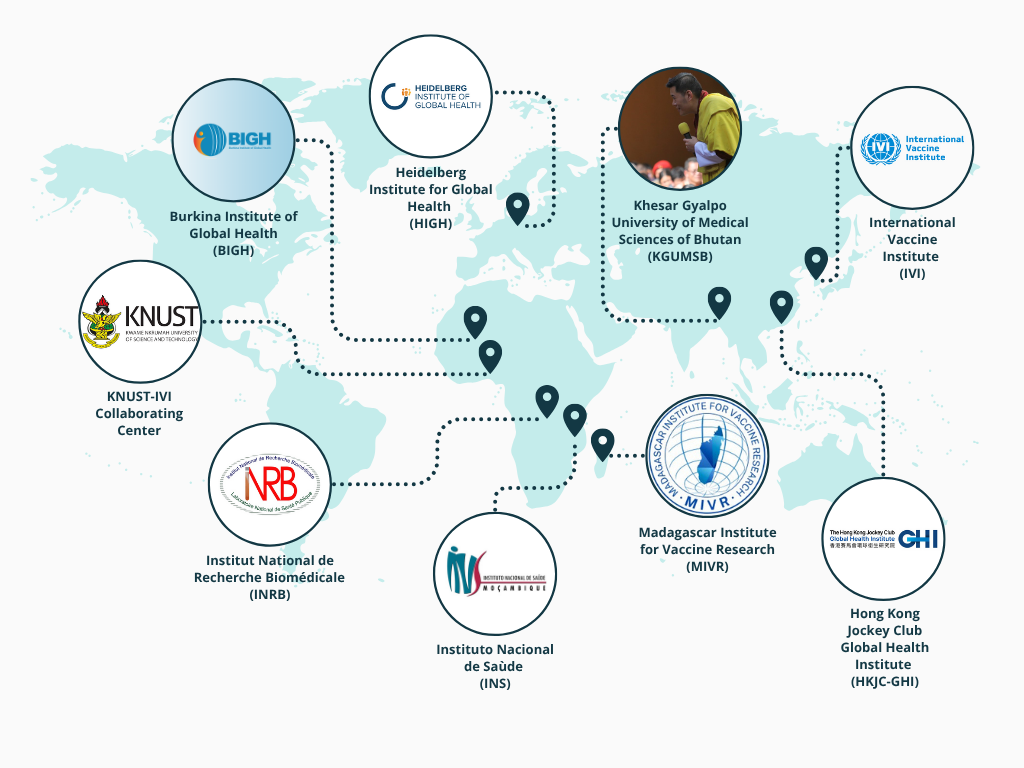
The Global Health team at CITIID works as part of a diverse, collaborative network of institutions advancing vaccine research, clinical trials, and public health capacity. Together with partners at the International Vaccine Institute (IVI), we have co-developed programs that combine scientific research with on-the-ground implementation across Africa, Asia, and globally.
Dr Florian Marks is the Deputy Director General of IVI’s Epidemiology, Public Health, and Impact (EPIC) unit. This unit plays a pivotal role in managing a diverse array of activities including comprehensive epidemiological investigations, undertaking cost-of-illness/cost-effectiveness studies, facilitating training and capacity improvement initiatives, exploring the impact of vaccines on antimicrobial resistance, as well as conducting post-licensure studies to assess vaccine effectiveness and impact.
Madagascar
We collaborate with the University of Antananarivo to establish an IVI collaborating center, the Madagascar Institute for Vaccine Research (MIVR), which has grown from a small team to over 100 staff and houses infrastructure for clinical trials and epidemiological studies. MIVR currently hosts key programs including:
- the Vaccine Against Schistosomiasis for Africa (VASA)
- the COVID-19 Research in African Settings (COVIA)
- the Severe Typhoid in Africa (SETA) Program
MIVR is part of the Typhoid Conjugate Vaccine Introduction in Africa program (THECA) consortium coordinated by the University of Cambridge, which received a €13 million grant from the European and Developing Countries Clinical Trials Partnership (EDCTP) in 2019 to assess the effectiveness of typhoid conjugate vaccine introduction in Ghana and the Democratic Republic of the Congo.
Ghana
We work with the Kwame Nkrumah University of Science and Technology (KNUST) to set up the KNUST-IVI collaborating center, which eventually to the formation of the Research Center for Global Health (RCGH) in Agogo, where studies such as the Typhoid Conjugate Vaccine Introduction in Africa (THECA) and Typhoid Vaccine Effectiveness in Ghana (TyVEGHA) are conducted.
Burkina Faso
We have been working jointly with the University of Ouagadougou / Institut Supérieur des Sciences de la Population (ISSP) since 2010. Jointly, we have Burkina Institute for Global Health (BIGH) that is actively supporting national typhoid surveillance and vaccine introduction efforts in partnership with IVI.
Bhutan
IVI is currently establishing a collaborating center with the Khesar Gyalpo University of Medical Sciences of Bhutan (KGUMSB), which will serve as a platform to support expanded research and public health activities in the country.
Democratic Republic of Congo (DRC) and Mozambique
The network also includes institutes in the Democratic Republic of Congo (DRC) and Mozambique; the Institut National de Recherche Biomédicale (INRB) and the Instituto Nacional de Saùde (INS), respectively. In both countries, large-scale effectiveness trials for typhoid fever and COVID-19 have been conducted alongside the implementation of health demographic surveillance (HDSS) sites.
Hong Kong
Culminating from these efforts, IVI, in partnership with the University of Hong Kong (HKU) and the University of Cambridge (UCAM), helped secure major funding to establish the Hong Kong Jockey Club Global Health Institute (HKJCGHI). Hosted at HKU, the institute brings together leading researchers from all three institutions and serves as a key node in the wider IVI global network. While based in Hong Kong, HKJCGHI is not limited to a regional focus; it plays an integral role in advancing vaccine process development, translational research, and workforce training across continents.
Cross-site collaborations
IVI and its partners actively coordinate across sites, holding regular joint planning meetings, technical exchanges, and South-South training activities. For instance, the Mozambican team supported rollout of a COVID-19 Phase 2 study in Madagascar, and country teams from Ghana, Madagascar, and Mozambique traveled to the DRC to support large-scale typhoid vaccine implementation.
EPIC at IVI also leads the African Regional Collaborative for Advanced Research Capacity in West Africa (ARC-WA), funded by the Coalition for Epidemic Preparedness Innovations (CEPI). ARC-WA supports a growing network of Good Clinical Practice (GCP)-compliant trial sites that contribute to both routine vaccine development and emergency response readiness. The initiative is coordinated in partnership with the Medical Research Council Unit The Gambia (MRCG) at the London School of Hygiene & Tropical Medicine (LSHTM), and works closely with regional and technical partners such as Africa CDC, WAHO, IQVIA, IAVI, and Edes & Associates.
Together, this global network of partnerships reflects a shared commitment to equitable vaccine access and sustainable research capacity. Through close collaboration across academic institutions, clinical sites, and public health agencies, IVI and its partners have built a connected ecosystem that bridges regions and disciplines. This collaborative model enables the sharing of expertise, infrastructure, and data across Africa, Asia, and other regions.
As the network continues to grow, there is a renewed focus on strengthening pandemic preparedness—not only in current areas of engagement but also through expanded efforts in Asia and other parts of the world. By aligning scientific innovation with coordinated action, the IVI network is positioned to support both immediate public health responses and long-term system resilience.

